Why Resin Designs?
Customer Centric |
Fast Prototyping |

Boutique Solutions |
About Resin Designs
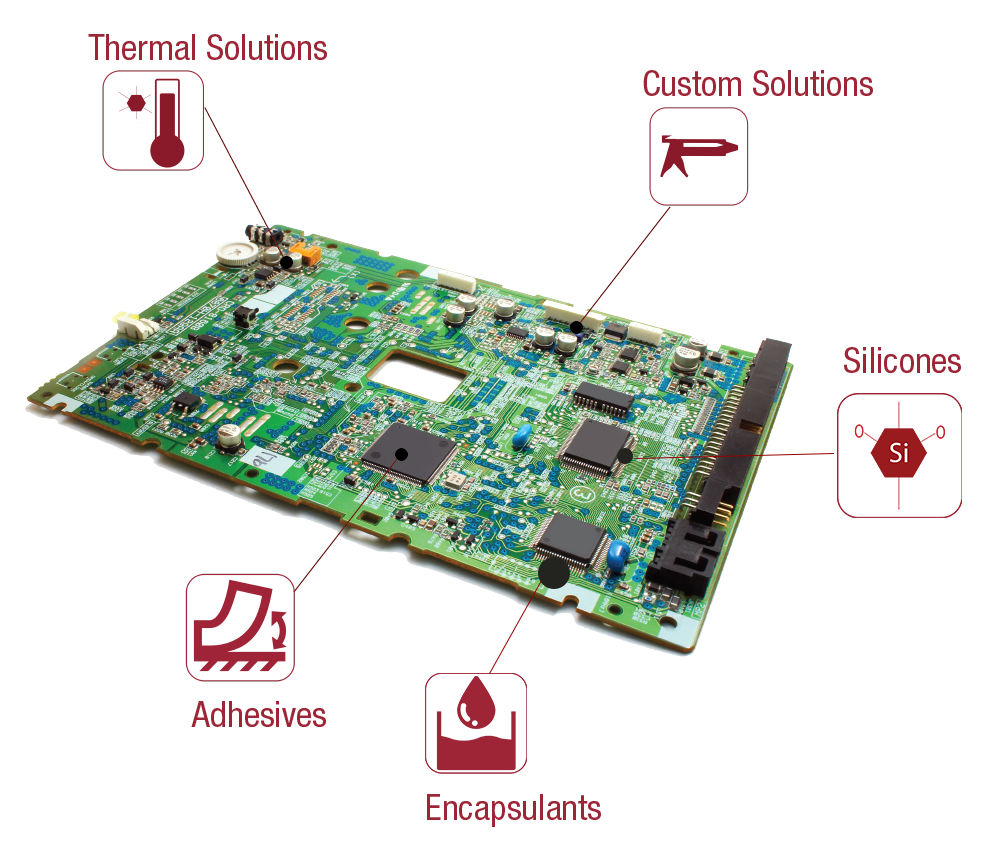
As an advanced adhesives and sealants manufacturer, Resin Designs develops and produces products for applications such as PCB Assembly and Enclosures, Medical Devices, Connector Sealing, EMI Shielding, Structural Bonding, and Thermal Management.
Available technologies include UV, single, and dual-component systems based on epoxy, urethane, and acrylate technologies. Other capabilities include pre-cured Silicone Gels, B-Staged Epoxy films, thermal management products, and rapid development of custom formulations.
Our facilities are fully ISO-9001 certified and our products are compliant to the latest RoHS and REACH regulations.
Quality & reliability you can count on … Don’t leave it to chance, leave it to Chase™
Let’s connect and never miss an update or news from us.